SYNTHETIC ORGANIC CHEMISTRY @HKU
Research Group of P Chiu
The University of Hong Kong

Publications
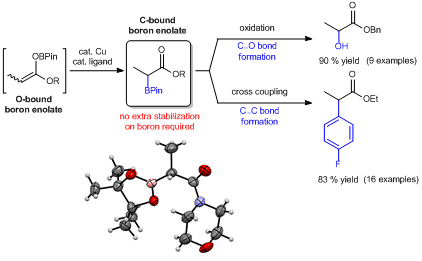
Synthesis and Applications of Unquaternized C-bound Boron Enolates
J. Am. Chem. Soc. 2018, xx, xx.
Abstract: A general and facile method to prepare unquaternized C-bound boron enolates by a ligand-controlled O-to-C isomerization is reported. Using this protocol, C-bound pinacolboron enolates have been isolated in pure form for the first time, and have been fully characterized by NMR spectroscopy and X-ray crystallography. In contrast to the general perception, such C-boron enolates are stable without coordinative saturation at the boron. Moreover, C-boron enolates present reactivities that are distinct from the O-boron enolates, and their applications in C‒O and C‒C bond formations are demonstrated.

Congratulations to Yu Chen and Diana Chan for their new paper in Chemistry-A European Journal!
Transannular [4+3] Cycloadditions of Macrocyclic Epoxy Ketones
ABSTRACT: Transannular [4+3] cycloadditions of dienophiles derived from macrocyclic epoxy ketones produce fused ring systems having central cycloheptane subunits. In some cases, base directly induced cycloisomerization of the epoxy ketones to yield the cycloadducts; in others, the epoxy ketones were transformed into their corresponding enolsilanes before undergoing cycloaddition. Enantiomerically-enriched tricyclic arrays are obtained from cycloadditions starting from optically pure epoxy ketones.

Diana will be working with Dr. Mike Porter at UCL in London, UK.

Congratulations to Ignatius Ip, Emily Lam and Dr. Zhengning Li for their new paper in Org. Lett!
Rearrangements of alpha-diazo-beta-hydroxyketones for the synthesis of bicyclo[m.n.1]alkanones
ABSTRACT: Rhodium-catalyzed decomposition of fused bicyclic a-diazo-b-hydroxyketones results in good yields of bridged bicyclo[m.n.1]ketones via a rearrangement pathway.

Congratulations to Emily Lam for her new paper in Org. Lett!
An approach to the welwistatin core via a diazoketone rearrangement-ring expansion strategy
ABSTRACT: The rhodium-catalyzed decomposition of fused bicyclic alpha-diazo-beta-hydroxyketone 16 and rearrangement to 17 is featured in an approach to the bridged bicyclic core of welwistatin. The bicyclic [4.3.1] core of 25 is furnished from a subsequent cyclopropanation to generate 23, followed by its ring expansion.