
SYNTHETIC ORGANIC CHEMISTRY @HKU
Research Group of P Chiu
The University of Hong Kong

Selected Publications
2022
-
Chemoselective and Diastereoselective Intramolecular (3+2) Cycloadditions of Epoxy and Aziridinyl Enolsilanes. Chen, Y.; Ling, J.; Keto, A. B.; He, Y.; Low, K. H.; Krenske, E. H.;* Chiu, P.* Angew. Chem. Int. Ed. 2022, e202116099.
Abstract: Epoxy and aziridinyl enolsilanes react as oxyallylic cation equivalents in highly chemo- and diastereoselective intramolecular (3+2) cycloadditions with a range of dienes and olefins. With acyclic dienes, the (3+2) cycloaddition outcompetes the (4+3) pathway traditionally observed in this kind of system almost exclusively. With both conjugated dienes and isolated olefins, excellent diastereoselectivities are observed, and cycloadducts can be obtained in optically-enriched forms. Computational studies indicate that the stepwise (3+2) cycloaddition involves an activated epoxy/aziridinyl intermediate and the conformational flexibility of the intermediate determines the preference for (3+2) cycloadduct formation. Further transformations of the (3+2) cycloadducts produce densely functionalized trans-hydrindane scaffolds.

-
Direct Asymmetric Reductive Amination of α-Keto Acetals: A Platform for Synthesizing Diverse α-Functionalized Amines. Shi, Y.; Wang, J.; Yang, F.; Wang, C.; Zhang, X.;* Chiu, P.; Yin, Q.* Chem. Commun. 2022, 58, 513-516. doi: 10.1039/D1CC06601C
Abstract: We report an efficient and straightforward method to synthesize enantio-enriched N-unprotected α-amino acetals via ruthenium-catalyzed direct asymmetric reductive amination. The α-amino acetal products are versatile and valuable platform molecules that can be converted to the corresponding α-amino acids, amino alcohols, and other derivatives by convenient transformations.

-
Copper-catalyzed Reductive Ireland-Claisen Rearrangements of Propargylic Acrylates and Allylic Allenoates. Guo, S.; Wong, K. C.; Scheeff, S.; He, Z.; Chan, W. T. K.; Low, K.-H.; Chiu, P.* J. Org. Chem. 2022, 87, 429-452. doi.org/10.1021/acs.joc.1c02455
Abstract: The copper-catalyzed reductive Ireland–Claisen rearrangement of propargylic acrylates led to 3,4-allenoic acids. The use of silanes or pinacolborane as stoichiometric reducing agents and triethylphosphite as a ligand facilitated the divergent and complementary selectivity for the synthesis of diastereomeric anti- and syn-rearranged products, respectively. Copper-catalyzed reductive Ireland–Claisen rearrangement of allylic 2,3-allenoates proceeded effectively only when pinacolborane was used as a reductant to generate various 1,5-dienes in excellent yields and with good diastereoselectivities in some cases. Mechanistic studies showed that the silyl and boron enolates, rather than the copper enolate, underwent a stereospecific rearrangement via a chairlike transition state to afford the corresponding Claisen rearrangement products.

2021
-
Copper-Catalyzed Enantioselective 1,2-Reduction of Cycloalkenones. Shi, Y. J.; Wang, J. X.; Yin, Q.; Zhang, X. M.*; Chiu, P.* Org. Lett. 2021, 23, 5658-5663. doi: 10.1021/acs.orglett.1c01744
Abstract: We report an asymmetric 1,2-reduction of cyclic α,β-unsaturated ketones to access various enantiomerically enriched cyclic allylic alcohols under mild conditions, catalyzed by in situ generated copper hydride ligated with (R)-DTBM-C3*-TunePhos. α-Brominated cycloalkenones were reduced with excellent enantioselectivities of up to 98% ee, while substrates that were without α-substituents were reduced chemoselectively, with moderate enantioselectivities.

2019
-
Pyrroles as Dienes in (4+3) Cycloadditions. Hu, F.; Ng, J. P. L.; Chiu, P.* Synthesis. 2019, 51, 1073-1086. doi: 10.1055/s-0037-1611660
Abstract: This Short Review summarizes the examples to date of successful (4+3) cycloadditions, including formal (4+3) cycloadditions, where pyrrole derivatives reacted as the diene component, to provide aza-bridged bicyclic and polycyclic adducts.

2018
-
Applications of (4+3) Cycloadditions in Natural Product Synthesis. Yin, Z. S.; He, Y.; Chiu, P.* Chem. Soc. Rev. 2018, 47, 8881-8924. DOI: 10.1039/C8CS00532J. Part of the themed collection: New Directions in Natural Product Synthesis.
Abstract: (4+3) Cycloadditions and its variants have been widely applied in synthesis. In this review article, we summarize some of more recent applications of (4+3) cycloadditions, including formal (4+3) cycloadditions, in the synthesis of natural products. Many of these natural product targets have cycloheptanoid frameworks for which the (4+3) cycloaddition is a convergent strategy for their assembly. Some natural product targets do not possess seven membered rings, and their syntheses have exploited the functional group endowed (4+3) cycloadducts resulting from these reactions, highlighting the utility of this methodology for the synthesis of many kinds of complex molecules.

-
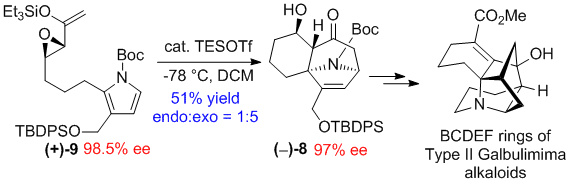
Intramolecular (4+3) Cycloadditions of Pyrroles and Application to the Synthesis of Class II Galbulimima Alkaloids. He, J.; Chen, Z.; Li, W.; Low, K.H.; Chiu, P.* Angew. Chem. Int. Ed. 2018, 57, 5253-5256. doi: 10.1002/anie.201711439
Abstract: The first intramolecular (4+3) cycloadditions with pyrroles to generate optically-enriched cycloadducts having the nortropane substructure occurred in good yields with epoxy enolsilanes as electrophiles. Using this pyrrole cycloaddition as the key step, we have achieved the asymmetric synthesis of the BCDEF rings common to the Class II galbulimima alkaloids.
-
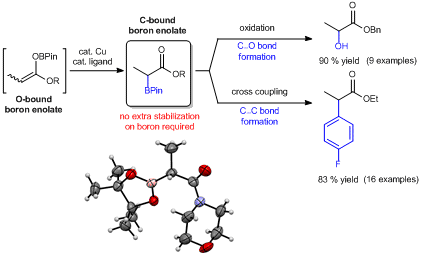
Synthesis and Applications of Unquaternized C-bound Boron Enolates. Ng., E. W. H.; Low, K.H.; Chiu, P.* J. Am. Chem. Soc. 2018, 140, 3537-3541. doi:10.1021/jacs.8b00614
Abstract: A general and facile method to prepare unquaternized C-bound boron enolates by a ligand-controlled O-to-C isomerization is reported. Using this protocol, C-bound pinacolboron enolates have been isolated in pure form for the first time, and have been fully characterized by NMR spectroscopy and X-ray crystallography. In contrast to the general perception, such C-boron enolates are stable without coordinative saturation at the boron. Moreover, C-boron enolates present reactivities that are distinct from the O-boron enolates, and their applications in C‒O and C‒C bond formations are demonstrated.
-
Application of a Rhodium-catalyzed Cyclization Cycloaddition Cascade Strategy to the Total Synthesis of (-)-Curcumol. Zhang, X. X.;* Ko, R. Y. Y.; Xie, X. Q.; Qi, W. P.; Li, P. C.; Chiu, P.* Org. Chem. Front., 2018, 5, 1092-1095. doi: 10.1039/C7QO01150D

-
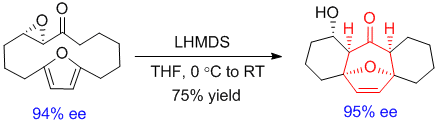
Transannular [4+3] Cycloadditions of Macrocyclic Epoxy Ketones. Chan, D.; Chen, Y.; Low, K. H.; Chiu, P.* Chem.-Eur. J. 2018, 24, 2375-2378. doi: 10.1002/chem.201800019/full
Abstract: Transannular [4+3] cycloadditions of dienophiles derived from macrocyclic epoxy ketones produce fused ring systems having central cycloheptane subunits. In some cases, base directly induced cycloisomerization of the epoxy ketones to yield the cycloadducts; in others, the epoxy ketones were transformed into their corresponding enolsilanes before undergoing cycloaddition. Enantiomerically-enriched tricyclic arrays are obtained from cycloadditions starting from optically pure epoxy ketones.

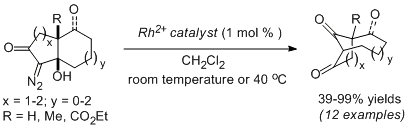
-
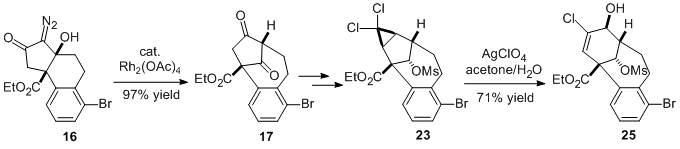
An Approach to the Welwistatin Core via a Diazoketone Rearrangement–Ring Expansion Strategy. Lam. S. M.; Wong, W. T.; Chiu, P.* Org. Lett. 2017, 19, 4468–4471. DOI: 10.1021/acs.orglett.7b01988
-
Rearrangements of α-Diazo-β-Hydroxyketones for the Synthesis of Bicyclo[m.n.1]alkanones. Li, Z.; Lam, S. M.; Ip, I.; Wong, W. T.; Chiu, P.* Org. Lett. 2017, 19, 4464–4467. DOI: 10.1021/acs.orglett.7b01963
-
A Natural Product-like JAK2/STAT3 Inhibitor Induces Apoptosis of Malignant Melanoma Cells. Wu, K. J.; Huang J. M.; Zhong, H. J.; Dong Z. Z.; Liu, C.; Lu, J.-J.; Chen, X. P.; Chiu, P; Kwong, D. W.; Han, Q. B.; Ma, D. L.*; Leung, C. H.* PLoS One, 2017, 12(6) e0177123. DOI: 10.1371/journal.pone.0177123
-
Dearomative Intramolecular (4+3) Cycloadditions of Arenes with Epoxy and Aziridinyl Enolsilanes. Ling, J.; Lam, S.; Low, K.-H.; Chiu, P.* Angew. Chem., Int. Ed. 2017, 56, 8879–8882. DOI: 10.1002/anie.201704155
-
Tetrandrine, an Activator of Autophagy, Induces Autophagic Cell Death via PKC-α Inhibition and mTOR-Dependent Mechanisms. Wong, V. K. W.; Zeng, W.; Chen J.; Yao, X. J.; Leung, E. L.; Wang Q. Q.; Chiu, P.*; Ko, B. C. B.*; Law, B. Y. K.* Front. Pharmacol. 2017, 8:351. DOI: 10.3389/fphar.2017.00351
2017
2016
-
3-Quinuclidinol. First Update. Y. Chen, Chiu, P.* In: Electronic Encyclopedia of Reagents for Organic Synthesis (e-EROS). John Wiley & Sons, 2016.
-
"Goldilocks Effect" of Water in Lewis-Brønsted Acid and Base Catalysis. Barron, B. J.; Wong, W. T.; Chiu, P.; Hii, K. K.* ACS Catalysis. 2016, 6, 4189–4194. DOI: 10.1021/acscatal.6b00800
-
Epoxy and Aziridinyl Enolsilanes in Diastereoselective Inter- and Intramolecular Friedel–Crafts Alkylations. Ling, J.; Lam, S. K.; Lo, B.; Lam, S.; Wong, W. T.; Chen, G.; Sun, J.; Chiu, P.* Org. Chem. Front. 2016, 3, 457–461. DOI: 10.1039/C5QO00333D.This article is selected to be in the themed collection: Hot articles in Organic Chemistry Frontiers in 2016.
-
Copper Hydride-Catalyzed Reductive Claisen Rearrangements. Wong, K. C.; Ng, E.; Wong, W. T.; Chiu, P.* Chem. – Eur. J. 2016, 22, 3709–3712. DOI: 10.1002/chem.201504870. This article is part of a Special Issue: Women in Chemistry.
2015
-
Formal Total Synthesis of (+)-Cortistatin A and J. Kuang, L.; Liu, L. L.; Chiu, P.* Chem. – Eur. J. 2015, 21, 14287–14291. DOI: 10.1002/chem.201502890. Selected as a Hot Paper by the Journal. Also highlighted in: Organic Chemistry Highlights: http://www.organic-chemistry.org/Highlights/2016/29August.shtm
-
Further studies on sultones derived from carbene cyclization cycloaddition cascades. Groß, T.; Herrmann, T.; Shi, B.; Jäger, A.; Chiu, P.; Metz, P.* Tetrahedron, 2015, 71, 5925–5931. DOI: 10.1016/j.tet.2015.05.095
-
Concerted Ring-Opening and Cycloaddition of Chiral Epoxy Enolsilanes with Dienes. Krenske, E. H.*; Lam, S.; Ng, J. P. L.; Lo, B.; Lam, S. K.; Chiu, P.*; Houk, K. N.* Angew. Chem., Int. Ed. 2015, 54, 7422–7425. DOI: 10.1002/anie.201503003
-
Cycloaddition Reactions of Carbonyl Ylides Derived From Enones. Yu, Y.; Cornelissen, L.; Wong, W. T.; Chiu, P.* Synlett 2015, 26 (11), 1553–1556. DOI: 10.1055/s-0034-1379926. Invited Contribution to Special Issue dedicated to Prof. K. C. Vollhardt.
-
Total Synthesis of (–)-Dolastatrienol. Leung, L. T.; Chiu, P.* Chem. – Asian J. 2015, 10, 1042–1049. DOI: 10.1002/asia.201403325. Selected as a VIP (Very Important Paper)
2014
-
Virtual screening and optimization of Type II inhibitors of JAK2 from a natural product library. Ma, D. L.*; Chan, D. S.-H.; Wei, G.; Zhong, H. J.; Yang, H.; Leung, L. T.; Gullen, E. A.; Chiu, P.*; Cheng, Y. C.*; Leung, C. H.* Chem. Commun. 2014, 50, 13885–13888. DOI: 10.1039/C4CC04498C
-
Vinyl Epoxides in Organic Synthesis. He, J.; Ling, J.; Chiu, P.* Chem. Rev. 2014, 114, 8037–8128. DOI: 10.1021/cr400709j
-
Intermolecular (4+3) cycloadditions of aziridinyl enolsilanes. Lam, S. K.; Lam, S.; Wong, W. T.; Chiu, P.* Chem. Commun. 2014, 50 (14), 1738–1741. DOI: 10.1039/C3CC48266A
-
[4+3] Cycloadditions of Enolsilane Derivatives. Lam, S. Y. Y.; Chiu, P.* In: Methods and Applications of Cycloaddition Reactions in Organic Synthesis (MACROS), N. Nishiwaki, Ed. Wiley Blackwell, 2014, Chapter 18, pp. 565–598.
-
Hexa-μ-hydrohexakis(triphenylphosphine)hexacopper. First Update. Chiu, P.*; Ng, W. H. In: Electronic Encyclopedia of Reagents for Organic Synthesis (e-EROS). John Wiley & Sons, 2014, pp. 1–9.
2010–2013
-
Asymmetric (4+3) Cycloadditions of Optically Enriched Epoxy Enolsilanes. Lo, B.; Lam, S.; Wong, W. T.; Chiu, P.* Angew. Chem., Int. Ed. 2012, 51, 12120–12123. DOI: 10.1002/anie.201207427. Selected as a Hot Paper by Angew. Chem., Int. Ed.
-
An Adventure in Synthesis Inspired by the Pseudolaric Acids. Chiu, P. In: Strategies and Tactics in Organic Synthesis, M. Harmata, Ed. Academic Press, 2012; Volume 8, Chapter 3, pp. 55–78.
-
Enantiomerically Enriched (4+3) Cycloadducts Derived from Optically Active Epoxy Enolsilanes. Lam, S.; Lo, B.; Wong, W. T.; Chiu, P.* Asian J. Org. Chem. 2012, 1, 30–33. DOI: 10.1002/ajoc.201200038. Selected as a VIP (Very Important Paper) and Front cover
-
Desymmetrizing reductive aldol cyclizations of enethioate derivatives of 1,3-diones catalyzed by chiral copper hydride. Ou, J.; Wong, W.-T.; Chiu, P.* Org. Biomol. Chem. 2012, 10, 5971–5978. DOI: 10.1039/C2OB25206F. Invited Contribution to the 10th Anniversary Issue of Org. Biomol. Chem.
-
Reductive aldol cyclizations of unsaturated thioester derivatives of 1,3-cyclopentanedione catalyzed by chiral copper hydrides. Ou, J.; Wong, W. T.; Chiu, P.* Tetrahedron, 2012, 68, 3450–3456. DOI: 10.1016/j.tet.2011.07.057. Invited Contribution to Tetrahedron Special Issue on “New trends in enantioselective catalysis with copper (I)” .
-
Desymmetrization of meso [3.2.1] oxabicyclic systems using metal-catalyzed asymmetric intramolecular C–H insertion. Zhang, X.; Li, Z.; Chu, J. C. K.; Chiu, P.* Tetrahedron Lett. 2011, 52, 6763–6766. DOI: 10.1016/j.tetlet.2011.10.026
-
Non-cross-linked polystyrene-supported triphenylarsonium halides and their use in the arsa-Wittig reaction. Lau, K. C. Y.; Chiu, P.* Tetrahedron, 2011, 67, 8769–8774. DOI: 10.1016/j.tet.2011.09.005
-
Conjugate reductions and reductive aldol cyclizations of α,β-unsaturated thioesters catalyzed by (BDP)CuH. Li, N.; Ou, J.; Miesch, M.; Chiu, P.* Org. Biomol. Chem. 2011, 9, 6143–6147. DOI: 10.1039/C1OB05352C
-
A Protecting-Group-Free Route to Chiral BINOL–Phosphoric Acids. Li, B.; Chiu, P.* Eur. J. Org. Chem. 2011, 3932–3937. DOI: 10.1002/ejoc.201100328
-
Synthesis of fluorinated analogues of the immunosuppressive drug FTY720. Ko, R. Y. Y.; Chu, J. C. K.; Chiu, P.* Tetrahedron, 2011, 67, 2542–2547. DOI: 10.1016/j.tet.2011.02.028
-
An expeditious asymmetric synthesis of the pentacyclic core of the cortistatins by an intramolecular (4+3) cycloaddition. Liu, L. L.; Chiu, P.* Chem. Commun. 2011, 47, 3416–3417. DOI: 10.1039/C1CC00087J. Selected as a Hot Article by Chem. Commun.
-
Facial Selectivity and Regiospecificity in the (4+3) Cycloaddition of Epoxy Enol Silanes. Lo, B.; Chiu, P.* Org. Lett. 2011, 13, 864–867. DOI: 10.1021/ol102897d
-
Pseudolaric Acids: Isolation, Bioactivity and Synthetic Studies. Leung, L. T.; Ko, B. C. B.; Chiu, P.* Nat. Prod. Rep. 2010, 27, 1066–1083. DOI: 10.1039/B906520M. Invited Review.
-
Alisol B, a Novel Inhibitor of the Sarcoplasmic/Endoplasmic Reticulum Ca(2+) ATPase Pump, Induces Autophagy,
Endoplasmic Reticulum Stress, and Apoptosis. Law, B. Y. K.; Wang, M.; Ma, D.-L.; Al-Mousa, F.; Michelangeli, F.; Cheng, S.-H..; Ng, M. H. L.; To, K.-F.; Mok, A. Y. F.; Ko, R. Y. Y.; Lam, S. K.; Chen, F.; Che, C.-M.; Chiu, P.*; Ko, B. C. B.* Mol. Cancer Ther. 2010, 69, 718–730. DOI: 10.1158/1535-7163.MCT-09-0700